The large-scale project M²OLIE (Mannheim Molecular Intervention Environment) has been receiving funding since 2013 as part of the BMFTR funding program “Research Campus – Public-Private Partnership for Innovation. funding program “Research Campus – Public-Private Partnership for Innovation.” The aim is to establish an optimized and gentle treatment pathway for cancer patients with oligometastases and to transfer the results into clinical practice.
The large-scale M²OLIE (Mannheim Molecular Intervention Environment) project has been supported since 2013 as part of the BMFTR funding program “Research Campus – Public-Private Partnership for Innovation.” The aim is to establish an optimized and gentle treatment pathway for cancer patients with oligometastases and to transfer the results into clinical practice.
The number of cancer patients worldwide and in Germany is expected to rise significantly in the coming decades. According to an estimate by the International Agency for Research on Cancer (IARC), the number of new cancer cases worldwide could increase to around 30 million by 2040 – a 47% increase compared to 2020. In Germany, the German Cancer Society and the Robert Koch Institute (RKI) predict an increase in cancer cases of around 10% to 15% by 2030. In addition to the increase in cancer cases, an increase in cancer deaths is also expected. Globally, the IARC estimates that around 16 million people could die of cancer each year by 2040 – an increase of around 40% compared to 2020. Experts in Germany anticipate a similar trend: according to the RKI, the number of cancer-related deaths is expected to rise by around 10% to 12% by 2030.
In the patient group participating in M²OLIE, the primary tumor is controllable, but there are also up to five metastases in one organ (e.g., the liver). Metastases are the leading cause of death in up to 70% of all cancer patients. Liver metastases are more common than primary liver tumors and are often associated with a poor prognosis compared to metastases in other locations: approximately 30% to 70% of cancer patients die as a result of liver metastases. Liver metastases often occur in advanced cancers, especially in colorectal carcinoma (40%–50%), breast cancer (50%–70%), lung cancer (13%–33%), pancreatic cancer (>40%), and stomach cancer (up to 34%). They develop due to the liver’s rich blood supply and the ability of tumors to spread through the bloodstream.
Currently, patients with oligometastases are predominantly treated palliatively. Despite knowledge of tumor heterogeneity (differences at the molecular genetic level), molecular analysis of all metastases in a patient is rarely performed. The individual steps of the usual treatment process for these patients, including purely manually assisted biopsy, histological analysis and diagnosis of metastasis, and therapy decision in the tumor board, which meets at most once a week, followed by inpatient stay, are spread over several weeks or even months. This is where M²OLIE’s closed-loop process concept for the curative treatment of this cancer comes in.
The focus of the work carried out at the M²OLIE Research Campus is the closed-loop process. This process combines diagnostics, therapy, and molecular analysis in a continuous, optimized workflow. Patients with oligometastases receive individualized, minimally invasive treatment of all tumor sites within a shorter period of time. State-of-the-art imaging, robot-assisted interventions, laboratory diagnostics, and case discussions in the tumor board are closely integrated to significantly increase precision and efficiency.
The public-private partnership with currently 25 partners unites the necessary interdisciplinary expertise under the umbrella of the University Hospital Mannheim. The long-term funding from the BMFTR for 15 years enables the implementation of a complex, holistic vision.
The closed-loop process of M²OLIE begins with patient admission (top left) and the generation of the electronic M²OLIE patient file. Then follows the electronic patient consultation, including digital signature, and the transfer of the case to the automated process control system with iterative processing of different treatment stages: multimodal (pre-)interventional imaging, robot-assisted biopsy, biopsy analysis using state-of-the-art mass spectrometry. Based on the comprehensive diagnostics, the ad hoc tumor board (top center) decides which minimally invasive therapy (i.e., surgery, interventional radiology, radiation therapy, radiopharmacy, see bottom center) should be performed. After the individualized therapy for each metastasis, the patient leaves the closed loop with or without subsequent drug-based tumor therapy (top right). Close follow-up care to promptly detect the recurrence of metastases is a standard part of the process. The blue area symbolizes the data lake, where all data is stored, processed, and made available as needed.
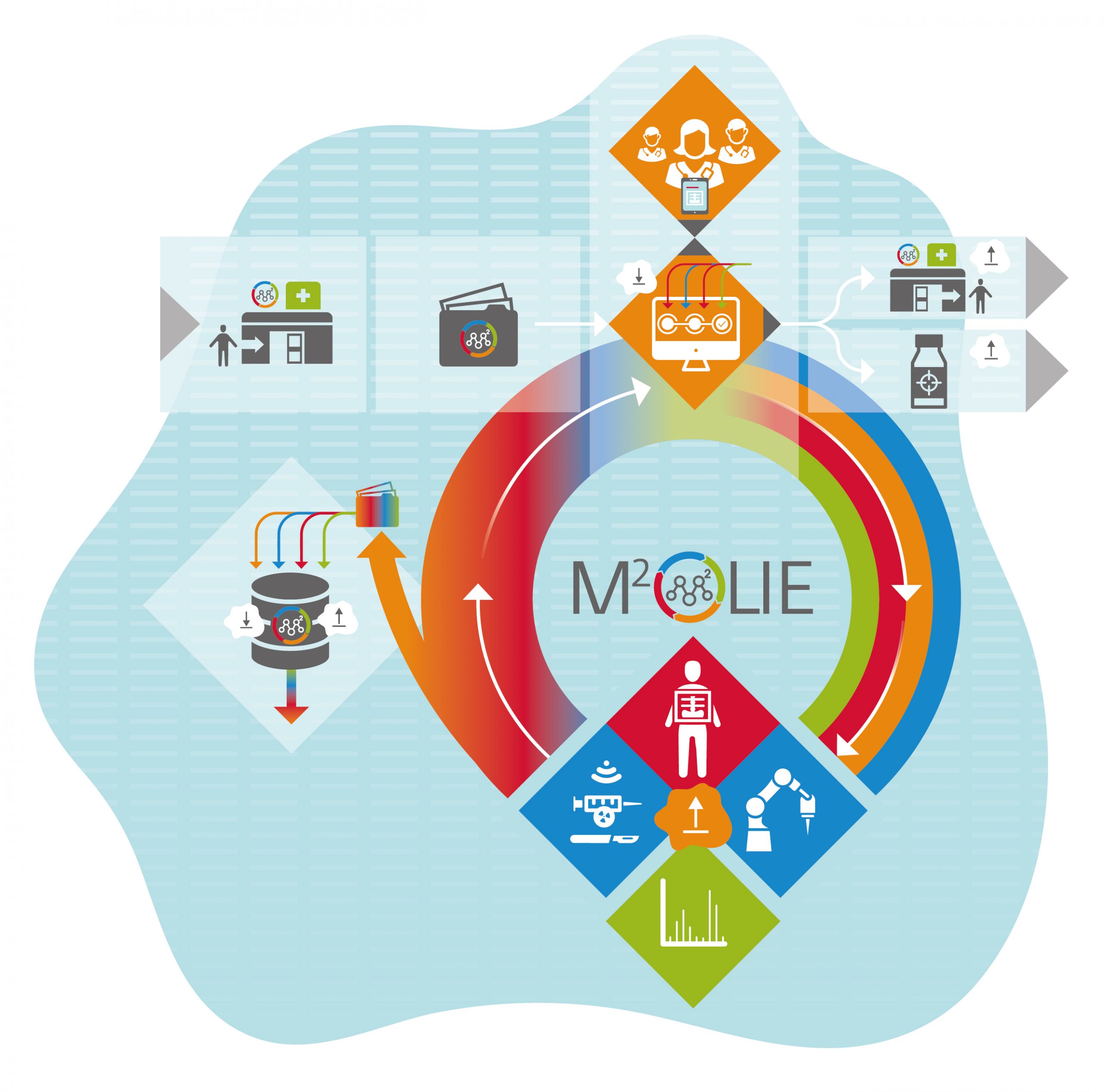
Graphic: The M²OLIE closed-loop process.
In the first funding phase, the focus was on establishing the technological and methodological foundations for the M²OLIE closed-loop process. The aim was to precisely research and link the individual steps of diagnosis, biopsy, and therapy. The joint projects M²IBID, M²INT, M²oBiTE, and the cross-sectional projects ProM²OLIE and M²PROCIT researched the basis for the time-optimized treatment pathway.
More about the 1st. funding phase
The second funding phase focused on integrating the individual steps into a continuous workflow, which was evaluated as a so-called basic process up to the biopsy. It was based on the joint projects M²IBID (imaging and molecular analysis), M²INT (automation and robotic assistance), and M²DATA (data supply process).
More about the 2nd. funding phase
In the third and final funding phase, the evaluated basic process from the second phase will be expanded into a comprehensive M²OLIE closed-loop process. The workflow will be further optimized in four modules (M²OLIE Operational Platform, M²OLIE Interventional Platform, M²OLIE Laboratory, M²OLIE Tumor Board). The results of these modules will form the basis for the planned M²OLIE clinic and transfer to clinical practice.
More about the 3rd. funding phase