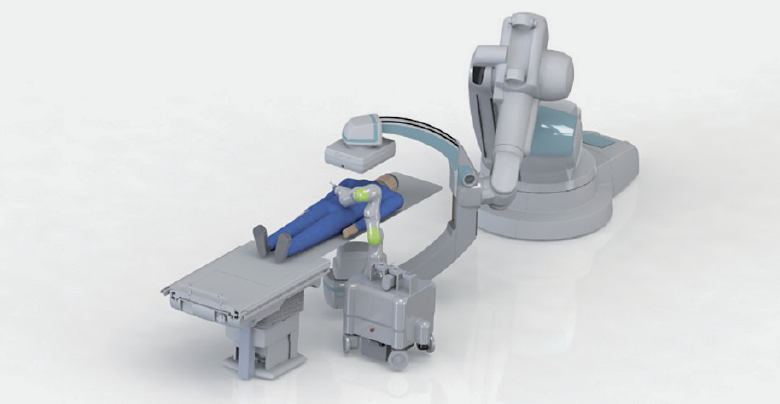

In view of the entire closed-loop process of M²OLIE, the joint project M²INT deals with the design and optimization of diagnostic and therapeutic processes in the intervention room. Effective treatment requires a compact, flexible and efficient sequence of diagnostic and therapeutic measures. Based on the results of the previous phase, the work in the second funding phase aims to remove existing limitations (such as efficiency barriers in the area of surgical set-up) and focus on marketable technologies for the design of future intervention rooms. The overall goal of the joint project M²INT is the integrated (further) development of medical methods, technical solutions and processes for the treatment of patients with oligometastases with methods of molecular medicine based on the closed-loop principle. At the end of the project, the partial solutions of M²INT are to be integrated into one system in the intervention room and to be evaluated in patients as part of the overall M²OLIE process in the clinic. The works within M²INT aim to increase the efficiency of the interventional process and improve the patient outcome as well as the intraoperative situation in the treatment of patients with oligometastases. The technological developments form the basis for the further development of the therapeutic applications, which in turn are to be used as a test task for the clinical evaluation of the technologies.
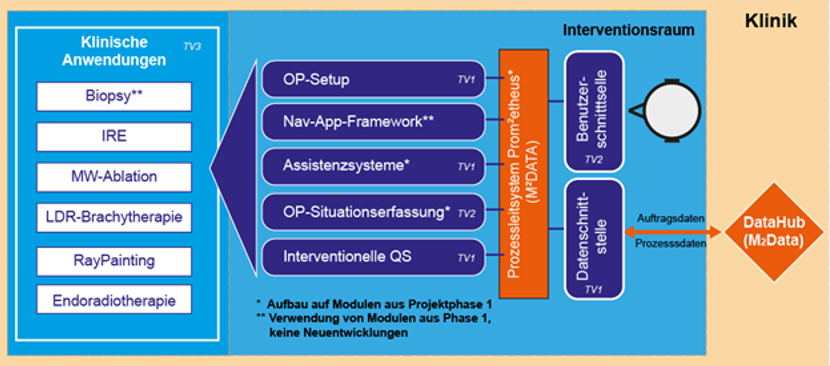
In order to achieve these goals, the M²INT joint project consists of three subprojects in which the necessary partial solutions are realized. In addition to the partial results, the overriding innovation is the high degree of integration of the overall system, including clinical evaluation. The projects partly build on the results of the first funding phase of M²INT or take them over entirely.
The first subproject “System platform for applications of molecular intervention” is coordinated by Fraunhofer IPA. In the first project phase it became clear that the efficient design of the overall process in the intervention room is crucial and serves as an essential prerequisite for the establishment of complex technologies. Therefore, this subproject deals with the research of methods and technologies to increase the efficiency of the OR setup and particularly the intervention-specific, automated patient positioning. An important goal is the further development of the assistance system for needle placement and its extension for the tele-manipulated and fully automated implementation of procedures based on needle probes. A system for interventional quality assurance will also be established as an important innovative element, which, with the aid of measuring probes, will initially allow conclusions to be drawn about the results of minimally invasive ablative therapies and, in future, enable model-based planning. Independent of the technological partial developments, the medical application development will be continued with the integration of all new partial solutions and the results from the first funding phase of M²OLIE. In detail, a robot-assisted procedure for the targeted intraoperative radiotherapy of brain tumors will be researched, a possibility for multimodal in vivo measurement of the clinical effect of minimally invasive ablative therapies will be established and the clinical translation of robot-assisted interventional procedures with multidetector computer tomographs (MDCT) and cone beam computer tomographs (CBCT) will be supported.








The second subproject “Navigated molecular diagnostics and therapy” is coordinated by the Emb-Lab of the Mannheim University of Applied Sciences. The subproject focuses on the development and implementation of methods for the automated situation recognition in the intervention room. For this purpose, the tracking solutions from the first phase for person recognition and the interpretation of situations in the intervention room will be further researched and completed using algorithms of machine learning. On this basis, a system to support patient positioning is being researched. Furthermore, the radiation dose in the room is visualized. The research of a tissue-differentiating biopsy needle already started in the previous phase will also be continued in clinical application by the Mannheim University of Applied Sciences.







The third subproject “Methods of Molecular Intervention” is coordinated by the Department of Radioation Oncology and the Institute of Clinical Radiology and Nuclear Medicine (IKRN) of the Mannheim Medical Faculty at the University of Heidelberg. In the first project phase of M²OLIE, a number of interventional methods for the use of assistance systems and for the application of closed-loop concepts were recommended. These applications will be evaluated from a medical point of view in clinical scenarios using the technologies from both funding phases of M²OLIE. For these applications, the processes for setting up the control system will be analyzed and new applications for molecular interventions identified and designed for the implementation as a closed-loop process. For the processes, a dataset will be determined with the help of which the results of the system evaluation in the intervention room, e.g. with regard to outcome and efficiency, can be evaluated. The results of the evaluation form an important basis for a potential commercial exploitation of the overall system concept. Furthermore, the third subproject supports partial tasks of the development of real-time personal dosimetry, dose distribution simulation for the robot-assisted radiotherapy of brain tumors as well as the development of a ground truth for the multimodal in vivo measurement and the research of possibilities of endoradiotherapy with functionalized gold nanoparticles and with 212Pb-labelled peptides to improve the outcomes of patients. In addition to the above-mentioned topics, the physicians of the University Medical Centre Mannheim make an indispensable contribution to the clinical translation of the developments through their commitment to the planning and execution of initial clinical experiments and studies with the developed technologies.



